News Details

Edited by Lynn Chiu
Author: Marco Treven (Department of Neurology, Medical University of Vienna; KLI fellow)
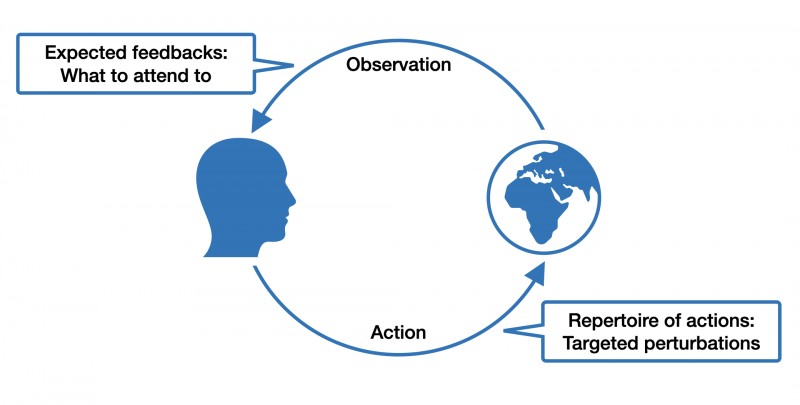
If you pick up Donald A. Norman’s famous book "The Design of Everyday Things," you will find a remarkable model outlined in Chapter two. It depicts a circular flow between an individual's goals and the world. This model starts with a prior goal, which informs actions, followed by perceiving and interpreting changes in the world, and finally, comparing the action outcome with the initial goal. Good design makes this iterative flow between goals and observable effects as smooth as possible: objects we use everywhere in our built human niche environment should "speak" to us about how to best interact with them.

Figure 2.2. copied from Norman (1998)
This is the gist of "affordances," a term coined by the ecological psychologist James J. Gibson. For example, if you see a door, you also perceive its "walk-through-ability." Affordance is not a phenomenon of the agent or the environment alone but emerges from the interaction between both. It follows that different organisms interact very differently with the same environmental features. For example, a hole in the ground might mean shelter for a mouse, but for a cat, it might mean food. When thinking in terms of affordances, it also becomes easier to appreciate the dynamic exchange between individuals and their built, social and natural environment as a dance between expectations, actions, and sensations.
What does the concept of affordances have to do with brain disorders?
In Parkinson’s disease, the gradual loss of dopamine-producing neurons leads to characteristic alterations of movement and cognition, which generally become slower, reduced in amplitude, rigid, and impoverished. There also appears to be something peculiar about how action opportunities (affordances) are perceived in Parkinson’s disease: freezing might occur when approaching a door, and a visual cue on the floor might help initiate the first step. One could conclude that if the environment is "speaking" to us, then the lack of dopamine in the brain makes it harder to understand what it is saying. Sometimes though, the message is loud and urgent enough to elicit a response. This would be the case of so-called "paradoxical kinesia," described already 100 years ago: "a Parkinson’s disease patient, confined to a wheelchair, will run out of a building at the sign of a fire."1
Is there an affordance network in the brain?
Parkinson's disease is increasingly described as one of several so-called circuitopathies. Circuitopathies are neuropsychiatric disorders characterized by specific constellations of altered brain network processes that correspond to sets of symptoms and phenomenology. According to this concept, multiple or distributed lesions may result in the same symptom if they are linked by a common functional or structural network, or they might impair the synchronized exchange of information between different networks. Framing neurological and psychiatric disorders as circuitopathies constitutes a major conceptual advancement in the understanding of these disorders and may also influence concrete treatment approaches. It can explain, for example, the current success of what is called "connectomic deep brain stimulation."
Now, one might ask which brain circuit is responsible for the apparent alteration of affordances in Parkinson's disease. At this point, a problem arises. Recall that affordances are a process of organism-environment interactions, so what exactly is the nature of the network we need to look at? Does the environment need to be accounted for, and if so, how? A common way to visualize and analyze brain networks is by methods such as tractography or functional MRI. Despite their success, utility, and improvements in temporal and spatial resolution, these methods emphasize networks within the confines of the skull of individual patients. They don't even capture the entire central nervous system, which also contains the spinal cord. Furthermore, it is easy to overlook that connections between neurons in the form of axons and synapses are not the only way brain networks are constructed. Extrasynaptic receptors, volume transmission, neuromodulators and hormones, as well as other non-neuronal cells, form and modulate network processes. Information exchange in the brain happens not just via landline but also wirelessly, so to speak. Additionally, multiple feedback loops of various reach exist not just between neurons and brain regions but also connect the brain to what is occurring in the body. This includes, among a myriad of parameters, information on muscle movements and joint positions fed back to the cerebellum for integration with motor intentions. It is difficult to appreciate the function of the cerebellum without considering feed-forward and feedback connections. In order to understand affordances, it seems that the network boundaries need to be extended a bit further yet. In a nutshell, some phenomena, such as altered affordances in PD, suggest that circuits can go beyond the brain.
Do brain networks extend beyond the brain?
It can be argued that brain feedback and control networks do not just extend beyond the central nervous system but also beyond the body as a whole. Take sensorimotor integration as an example: similar to muscle movements and joint positions, the changes in visual sensations that follow a turn of the head are subject to prior predictions, creating another feedback loop. There is not much controversy about the notion that perception is shaped by prior expectation, going back at least to Helmholtz’s "perception as inference." It is interesting in this regard that the strength of optical illusions can vary substantially in different neuropsychiatric disorders.
Another example of extended networks is the synchronized brain activity of interacting individuals in EEG hyperscanning experiments2, as well as, e.g., synchronization of bodily movements3. There may be methodological pitfalls, but it appears that just like information transfer between two brain areas requires synchronization, the same is true for social interactions. It is also well known that social networks and neuropsychiatric conditions have strong bi-directional influences on each other4. Thus, the (subconscious) perception of subtle action opportunities seems necessary for this kind of coupling, and more work should go into understanding how our ability to synchronize with others is influenced by various brain disorders.
Could the "active inference" framework help to understand certain phenomena of circuitopathies?
If the brain really is a self-organizing "good regulator" and, therefore, a model of its environment, like the so-called "active inference" approach suggests5, then this needs to be taken seriously when addressing circuit disorders. Active inference is a framework describing the adaptive, reciprocal interaction between living organisms and their volatile environments. Actions emitted based on prior experiences predict resulting sensory observations in a manner that helps to make sense of the surrounding world. Such observations may correspond to desired outcomes or goals. Both sides of this action-perception loop require selection and reinforcement processes. This enactive approach to brain function still has a long way to arrive in clinical practice fully, and there are open questions about the structural correlates of the cognitive theory in actual brain networks.
In our recent collaborative hypothesis article (Spee et al.6), we combined inputs from cognitive theory, psychology, aesthetics, and clinics, to find systematic patterns of how various disorders affecting the so-called “basal ganglia loop” in the brain, such as Parkinson's Disease, influence fundamental aspects of perceiving, interacting, and expressing oneself within changing environments6. It should be emphasized that this theoretical work takes the liberty of speculation, but there nevertheless appears to be an intriguing alignment between neuropathology, phenomenology, and accounts of neuropsychiatric disorders that treat the brain as a predictive organ. Discrepancies between predictions and observations, either due to particularly volatile environments or due to changes in the brain's apparatus for processing and weighting observations, may manifest in discernible behavioral phenomena. This could have potential implications for future, more rigorous investigations and how to put brain-centered treatment methods (e.g., pharmacological or surgical) in relation to environment-centered methods (e.g., cueing devices, music-, art-, or group-based therapies).
Conclusion
Looking at the brain as a predictive organ resembles Norman's design model for interacting with everyday things, painting the picture of a circular dynamic exchange between an individual's goals/expectations and the surrounding world. Learning and adapting entails continuous hypothesis testing by playfully generating and resolving errors of prediction. In the motor domain (which is most visibly affected in Parkinson's disease), this means that fluid, sequenced, and coordinated movements require the processing of an array of fluctuating proprioceptive but also exteroceptive prediction errors, meaning differences between intended and actual motor-sensory states. This suggests that some phenomena of circuitopathies, such as altered affordances, are not realized by networks within the brain alone but are better depicted by including loops of sensorimotor integration to reflect the role of the built, social and natural environment.
References:
1. Souques, M. A. Repport sur les syndromes parkinsoniens. Rev. Neurol. 1, 534–573 (1921).
2. Pérez, A., Carreiras, M. & Duñabeitia, J. A. Brain-to-brain entrainment: EEG interbrain synchronization while speaking and listening. Sci. Rep. 7, 4190 (2017).
3. Tschacher, W., Rees, G. M. & Ramseyer, F. Nonverbal synchrony and affect in dyadic interactions. Front. Psychol. 5, 1323 (2014).
4. Dhand, A., Luke, D. A., Lang, C. E. & Lee, J.-M. Social networks and neurological illness. Nat. Rev. Neurol. 12, 605–612 (2016).
5. Parr, T., Pezzulo, G. & Friston, K. J. Active Inference: The Free Energy Principle in Mind, Brain, and Behavior. (MIT Press, 2022).
6. Spee, B. T. M. et al. Repeating patterns: Predictive processing suggests an aesthetic learning role of the basal ganglia in repetitive stereotyped behaviors. Front. Psychol. 13, (2022).
About the Author:
Marco Treven is an MD/Ph.D. and resident at the Department of Neurology, Medical University of Vienna (MUW). He received his Ph.D. in Neurobiology from the MUW with a dissertation about extrasynaptic GABA(A) receptors in neurological disorders and basal ganglia. Building on this pharmacological work, he has subsequently developed a particular focus on movement disorders and deep brain stimulation. He is interested in deliberate, automatic, and repetitive human behavioral patterns, expressed in movement and abstract cognition, and how such patterns are related to generative internal models or beliefs. In this context, he aims to expand the cognitive framework of Predictive Processing in neurological disorders and extended/distributed cognition. This includes the notion of individually and culturally shaped affordances and the principles of active inference, prediction error minimization, and determinants of belief revision. The potential relevance of this work extends from a better understanding of neuropsychiatric disorders to the interplay between public health and sustainable behavior within environmental niches.
Learn more about his KLI project here: Applying Predictive Processing to Behavioural Patterns in Neuropsychiatric Disorders and Distributed Cognition

